Introduction: There is an unmet need to examine anti-tumor immune responses and predictive biomarkers in the peripheral blood to guide effective combination immunotherapies in classic Hodgkin Lymphoma (cHL). We hypothesized that treatment with the anti-CD30 antibody drug conjugate brentuximab vedotin (Bv) in combination with chemotherapy will have dual therapeutic effects in HL, with direct antiproliferative effects and a restoration of favorable tumor specific cytotoxic T cell immune response. We evaluated tumor-specific cytotoxic T cell response, cytokine and chemokine milieu in the peripheral blood of pediatric patients with HL before and after treatment with either standard chemotherapy or chemotherapy + Bv.
Methods: Children's Oncology Group clinical trial AHOD1331 was a randomized phase III trial for newly diagnosed high risk cHL ages ≥2 to 22 years which compared standard chemotherapy with doxorubicin, bleomycin, etoposide, prednisone and cyclophosphamide (ABVE-PC) to Bv + AVE-PC with response adapted radiation. Peripheral blood samples were collected at diagnosis (pre) and following completion of treatment (post). Peripheral blood mononuclear cells and plasma were isolated using Ficoll Density gradient. Plasma was cryopreserved for batch testing of Th1/Th2 cytokines, sCD30, sCD163 and TARC by Luminex assay. Cytotoxic T cell responses to non-EBV HL specific tumor associated antigens (TAAs) MAGEA4, PRAME, and survivin pre and post treatment were tested using the interferon-γ ELISPOT assay after ex vivo expansion. TAA-T cell specificity was reported as spot forming count (SFC per 10 5 T cells). Changes in cytokine levels and T cell responses pre versus post treatment were compared using paired t-tests and blinded to treatment arm.Levels of sCD30, sCD163 and TARC were compared both pre and post treatment. Thecox model was used to evaluate association between levels and event free survival (EFS) across both arms.
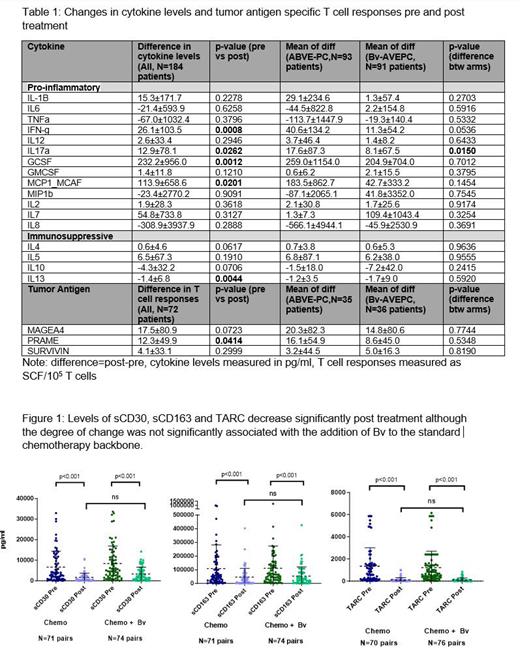
Results There was no difference in patient or disease characteristics between the entire study cohort (n=587) and patients analyzed (n=216). There were 184 patients with pre and post cytokine data, 71 patients with pre and post ELISPOT-T cell data, 147 patients with pre and post sCD30/sCD163 data, and 146 with pre and post TARC data. Compared to pre-treatment levels, there was a significant reduction in the immunosuppressive cytokines IL-13 (p=0.004) and increase in pro-inflammatory cytokines IL17a (p=0.03), Interferon-γ (p=0.0008), GCSF(p=0.0012) and MCP1(p=0.02). This change in cytokine levels from baseline to post-treatment was similar for both treatment arms except a higher increase in IL17a(p=0.015) was noted in the standard chemotherapy arm compared to the Bv arm (Table 1). Among 147 paired samples evaluated, sCD30, sCD163 and TARC all were significantly lower post treatment (Figure 1), but did not differ across arms. Pre treatment sCD163 and TARC values were significantly associated with EFS (p = 0.005 and 0.045), as was the degree of change in sCD163 post-treatment levels as compared to pre-treatment levels (p = 0.0156). Among 71 patients with pre and post ELISPOT-T cell data, there was a significant increase in the T cell responses specific to PRAME(p=0.04) post treatment and a trend for increased MAGEA4, but no association with EFS.
Conclusions Treatment results in an increased T cell response to HL-specific antigen PRAME post therapy in both treatment arms suggesting that recovery post anti-HL treatment can promote tumor antigen specific T cell immunity in vivo. Further, reduction in immunosuppressive cytokines IL-13 and changes in proinflammatory cytokines Interferon-γ following treatment suggest a favorable milieu for T cell expansion. Pre-treatment levels of soluble CD163 and TARC are associated with EFS and may present a potential predictive biomarker, as well as degree of change of CD163 post treatment, irrespective of addition of immunotherapy. Our results have implications for identifying immune markers of response to guide future immunotherapies and cytotoxic T cell therapy in cHL. (NCT02166463)
Disclosures
Dave:Merck: Current Employment. Horton:Takeda: Research Funding. Kelly:Seagen: Other: Scientific Steering Committee; Merck: Other: Scientific Steering Committee. Roth:Merck: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees. Castellino:Bristol Meyers Squibb: Honoraria, Other: Scientific Advisory Committee; SeaGen Inc.: Other: Scientific Advisory Committee - No honoraria, Research Funding. Bollard:Cabaletta Bio, Catamaran Bio: Current equity holder in private company, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: Patent applications in CAR-NKs; Roche: Consultancy.